A chiral BINOL-based Gemini amphiphilic gelator and its specific discrimination of native arginine by gelation in water†
Abstract
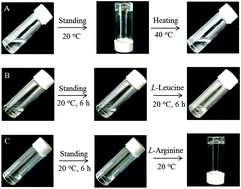
A novel axially chiral cationic Gemini amphiphile gelator (S1) derived from (S)-BINOL has been synthesized and characterized by 1H NMR, 13C NMR, ESI-MS and FT-IR analyses. The critical micelle concentration (CMC) of S1 was determined to be 0.21 mM in water at room temperature. A transparent hydrogel with S1 at 43 mM was obtained at room temperature and characterized using various methods including SEM, CD, fluorescence, 1H NMR, FT-IR, and XRD. The results indicate that the hydrophobic effect of long alkyl chains, π–π stacking of naphthalene rings, and intermolecular hydrogen-bonding of the amide groups of S1 should be responsible for the hydrogel formation. Moreover, an 8.5 mM aqueous solution of S1 could gel by the addition of L-arginine, whereas it failed to gel in the presence of other 15 amino acids, respectively. It is suggested that S1 could discriminate native arginine by hydrogel formation, mainly due to the electrostatic interaction and hydrogen bonding effects between S1 and L-arginine molecules.


 Please wait while we load your content...
Please wait while we load your content...