Reversible helical chirality of perylene bisimide aggregates: amino acid-directed chiral transfer and chiral inversion†
Abstract
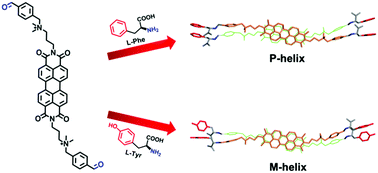
Through the formation of dynamic covalent bonds, we succeeded, for the first time, in achieving a reversible chiral transfer from amino acids to perylene bisimide aggregates in aqueous solutions. Two opposite helical aggregations are induced with L-phenylalanine and L-tyrosine, respectively. It is possible that the change in configurations of phenyl groups in amino acids leads to the chiral inversion of BAPBI arrangements.


 Please wait while we load your content...
Please wait while we load your content...