Sugar-coated proteins: the importance of degree of polymerisation of oligo-galacturonic acid on protein binding and aggregation
Abstract
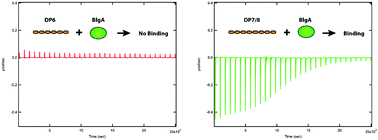
We have simplified the structural heterogeneity of protein–polysaccharide binding by investigating protein binding to oligosaccharides. The interactions between bovine beta-lactoglobulin A (βLgA) and oligo-galacturonic acids (OGAs) with various numbers of sugar residues have been investigated with a range of biophysical techniques. We show that the βLgA–OGA interaction is critically dependent on the length of the oligosaccharide. Isothermal titration calorimetry results suggest that a minimum length of 7 or 8 sugar residues is required in order to exhibit appreciable exothermic interactions with βLgA – shorter oligosaccharides show no enthalpic interactions at any concentration ratio. When titrating βLgA into OGAs with more than 7–8 sugar residues the sample solution also became turbid with increasing amounts of βLgA, indicating the formation of macroscopic assemblies. Circular dichroism, thioflavin T fluorescence and small angle X-ray/neutron scattering experiments revealed two structural regimes during the titration. When OGAs were in excess, βLgA formed discrete assemblies upon OGA binding, and no subsequent aggregation was observed. However, when βLgA was present in excess, multi-scale structures were formed and this eventually led to the separation of the solution into two liquid-phases.


 Please wait while we load your content...
Please wait while we load your content...