Confinement effect on the structure and elasticity of proteins interfacing polymers†
Abstract
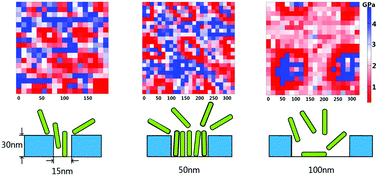
The ordered nanostructured surfaces provide confined environments that allow functionalization of proteins. Here, we used the nanopores of poly(methyl methacrylate) films to attach fibrinogen and lysozyme, and discussed the changes in proteins' structures and elasticity upon confinement. Fourier-transform infrared spectroscopic analysis of protein secondary structures reveals that fibrinogen undergoes less structural change and behaves less stiff when the pore size is close to the protein size. Lysozyme, on the other hand, retains its native-like structure, however, it exhibits the highest modulus in 15 nm pores due to the lower macromolecular crowding effect the protein faces compared to lysozyme within larger pores. These findings manifest the effect of confinement and crowding on the conformation and elasticity of different shaped proteins tethered on surfaces.


 Please wait while we load your content...
Please wait while we load your content...