Polymer glass transition occurs at the marginal rigidity point with connectivity z* = 4
Abstract
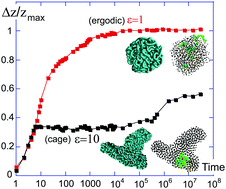
We re-examine the physical origin of the polymer glass transition from the point of view of marginal rigidity, which is achieved at a certain average number of mechanically active intermolecular contacts per monomer. In the case of polymer chains in a melt/poor solvent, each monomer has two neighbors bound by covalent bonds and also a number of central-force contacts modelled by the Lennard-Jones (LJ) potential. We find that when the average number of contacts per monomer (covalent and non-covalent) exceeds the critical value z* ≈ 4, the system becomes solid and the dynamics arrested – a state that we declare the glass. Coarse-grained Brownian dynamics simulations show that at sufficient strength of LJ attraction (which effectively represents the depth of quenching, or the quality of solvent) the polymer globule indeed crosses the threshold of z*, and becomes a glass with a finite zero-frequency shear modulus, G ∝ (z − z*). We verify this by showing the distinction between the ‘liquid' polymer droplet at z < z*, which changes shape and adopts the spherical conformation in equilibrium, and the glassy ‘solid’ droplet at z > z*, which retains its shape frozen at the moment of z* crossover. These results provide a robust microscopic criterion to tell the liquid apart from the glass for the linear polymers.


 Please wait while we load your content...
Please wait while we load your content...