Effects of molecular chirality on self-assembly and switching in liquid crystals at the cross-over between rod-like and bent shapes†
Abstract
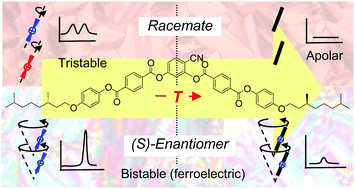
A bent-core compound derived from a 4-cyanoresorcinol core unit with two terephthalate based rod-like wings and carrying chiral 3,7-dimethyloctyloxy side chains has been synthesized in racemic and enantiomerically pure form and characterized by polarizing microscopy, differential scanning calorimetry, X-ray diffraction and electro-optical investigations to study the influence of molecular chirality on the superstructural chirality and polar order in lamellar liquid crystalline phases. Herein we demonstrate that the coupling of molecular chirality with superstructural layer chirality in SmCsPF domain phases (forming energetically distinct diastereomeric pairs) can fix the tilt direction and thus stabilize synpolar order, leading to bistable ferroelectric switching in the SmC* phases of the (S)-enantiomer, whereas tristable modes determine the switching of the racemate. Moreover, the mechanism of electric field induced molecular reorganization changes from a rotation around the molecular long axis in the racemate to a rotation on the tilt-cone for the (S)-enantiomer. At high temperature the enantiomer behaves like a rod-like molecule with a chirality induced ferroelectric SmC* phase and an electroclinic effect in the SmA′* phase. At reduced temperature sterically induced polarization, due to the bent molecular shape, becomes dominating, leading to much higher polarization values, thus providing access to high polarization ferroelectric materials with weakly bent compounds having only “weakly chiral” stereogenic units. Moreover, the field induced alignment of the SmCsPF(*) domains gives rise to a special kind of electroclinic effect appearing even in the absence of molecular chirality. Comparison with related compounds indicates that the strongest effects of chirality appear for weakly bent molecules with a relatively short coherence length of polar order, whereas for smectic phases with long range polar order the effects of the interlayer interfaces can override the chirality effects.

 Please wait while we load your content...
Please wait while we load your content...