Two bent-shaped π-organogelators: synthesis, fluorescence, self-assembly and detection of volatile acid vapours in gel films and in gel–gel states†
Abstract
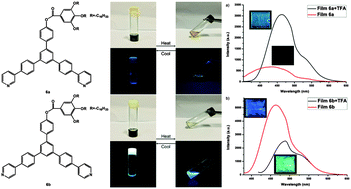
Two novel bent-shaped π-organogelators 6a and 6b having different terminal pyridine rings as responsive sites were designed, synthesized and fully characterized. A subtle difference in the position of the N atom at the pyridine ring greatly affected their fluorescence and gelation properties. 6b showed remarkably stronger fluorescence both in solution and in the solid state as compared to 6a. Theoretical calculation revealed a clear discrepancy in the electron distribution between them. Furthermore, driven by π–π stacking interaction and hydrophobic interaction, both 6a and 6b can gelate several organic solvents with different polarities. Rheological studies, spectroscopic tests and powder X-ray diffraction showed that 6a displayed a closer stacking mode leading to stronger gel robustness. The xerogel films of 6a and 6b were prepared and utilized to detect acid vapours. Both of them can fulfil the detection of acid vapours through a distinct fluorescence change which could be seen by the naked eye under a UV lamp, but with different sensing modes. A rare gel to gel transformation was also observed upon exposure to acid vapours accompanied by a morphological change.

 Please wait while we load your content...
Please wait while we load your content...