Overcoming inactivation of the lung surfactant by serum proteins: a potential role for fluorocarbons?
Abstract
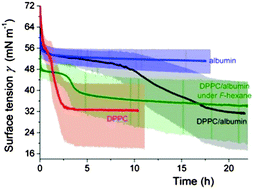
In many pulmonary conditions serum proteins interfere with the normal adsorption of components of the lung surfactant to the surface of the alveoli, resulting in lung surfactant inactivation, with potentially serious untoward consequences. Here, we review the strategies that have recently been designed in order to counteract the biophysical mechanisms of inactivation of the surfactant. One approach includes protein analogues or peptides that mimic the native proteins responsible for innate resistance to inactivation. Another perspective uses water-soluble additives, such as electrolytes and hydrophilic polymers that are prone to enhance adsorption of phospholipids. An alternative, more recent approach consists of using fluorocarbons, that is, highly hydrophobic inert compounds that were investigated for partial liquid ventilation, that modify interfacial properties and can act as carriers of exogenous lung surfactant. The latter approach that allows fluidisation of phospholipid monolayers while maintaining capacity to reach near-zero surface tension definitely warrants further investigation.

 Please wait while we load your content...
Please wait while we load your content...