Chemical modification of Nafion membranes by protic ionic liquids: the key role of ionomer–cation interactions†
Abstract
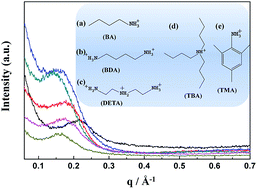
Chemically modified Nafion composite membranes were successfully fabricated using five kinds of protic ionic liquids (PILs) with different cations, 1-butylammonium methanesulfonate (BA-MS), tributylammonium methanesulfonate (TBA-MS), 2,4,6-trimethylphenylammonium methanesulfonate (TMA-MS), butane-1,4-diammonium methanesulfonate (BDA-MS), and N-(2-aminoethyl)ethane-1,2-diammonium methanesulfonate (DETA-MS). The PIL incorporated Nafion composite membranes were characterized by impedance spectroscopy, small-angle X-ray scattering (SAXS), dynamic-mechanical analysis (DMA) and thermogravimetric analysis (TGA). In general, the Nafion/PIL composite membranes exhibit a significant increase in the ionic conductivities than Nafion under anhydrous conditions. The interactions between the Nafion ionomer and different geometric cations of PILs were also discussed by the comparison of nanostructures, dynamic-mechanical properties and thermal stabilities of the Nafion/PIL composite membranes.

 Please wait while we load your content...
Please wait while we load your content...