Solvent dependent interactions between droplets in water-in-oil microemulsions
Abstract
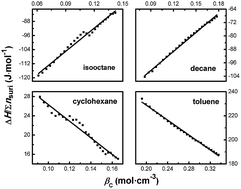
In this paper, we investigated the dilution enthalpies of the droplets in water/AOT/oil microemulsions with oil being isooctane, decane, or cyclohexane by isothermal titration microcalorimetry (ITC). Combining with the results obtained from the study of the water/AOT/toluene system in our previous work, it was found that the enthalpy interactions between droplets for isooctane and decane systems were repulsive, while the enthalpy interactions were attractive for cyclohexane and toluene systems. The repulsive droplet interaction for the isooctane system was also confirmed by static light scattering. The solvents appear to play important roles in varying the droplet enthalpy interactions from positive to negative, and the entropy contribution seems to be dominant for the stability of these microemulsion droplet systems.

 Please wait while we load your content...
Please wait while we load your content...