One role of hydration water in proteins: key to the “softening” of short time intraprotein collective vibrations of a specific length scale
Abstract
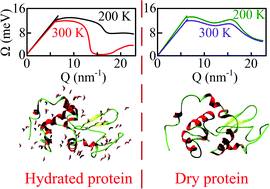
High resolution inelastic X-ray scattering (IXS) experiments show that the “phonon energy softening” and “phonon population enhancement” observed in a hydrated native protein when increasing the temperature from 200 K to physiological temperature are not directly related to the protein structure. Such phenomena were also observed in a denatured sample without a defined tertiary structure and with a limited residual secondary structure. However, in a dry sample, such “softening” is strongly suppressed. These facts suggest that the above-mentioned protein “softening” phenomenon is water-induced. In addition, increasing the hydration level can also induce “phonon energy softening” at room temperature, but not at 200 K. This change may be due to a qualitative difference in the dynamics of hydration water at 200 K and at room temperature.

 Please wait while we load your content...
Please wait while we load your content...