How ions in solution can change the sign of the critical Casimir potential
Abstract
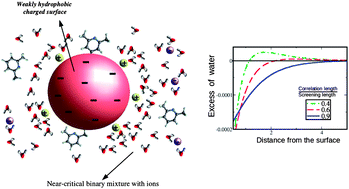
We show that hydrophilic ions present in a confined, near-critical aqueous mixture can lead to an attraction between like charge surfaces with opposing preferential adsorption of the two species of the mixture, even though the corresponding Casimir potential in uncharged systems is repulsive. This prediction agrees with a recent experiment [Nellen et al., Soft Matter, 2011, 7, 5360]. We also show that oppositely charged hydrophobic surfaces can repel each other, although the Casimir potential between uncharged surfaces with like preferential adsorption (selectivity) is attractive. This behavior is expected when the electrostatic screening length is larger than the correlation length, and one of the confining surfaces is strongly selective and weakly charged, whereas the other confining surface is weakly selective and strongly charged. The Casimir potential can change sign because the hydrophilic ions near the weakly hydrophobic surface can overcompensate the effect of hydrophobicity, and this surface can act as a hydrophilic one. We also predict a more attractive interaction between charged, hydrophilic surfaces and a more repulsive interaction between charged, hydrophobic surfaces than given by the sum of the Casimir and Debye–Hückel potentials. Our theory is derived systematically from a microscopic approach, and combines the Landau-type and Debye–Hückel theories with an additional contribution of an entropic origin.

 Please wait while we load your content...
Please wait while we load your content...