Non-equilibrium effects evidenced by vibrational spectra during the coil-to-globule transition in poly(N-isopropylacrylamide) subjected to an ultrafast heating–cooling cycle†
Abstract
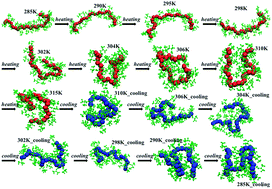
Molecular dynamics simulations in conjunction with finite element calculations are used to explore the conformational dynamics of a thermo-sensitive oligomer, namely poly(N-isopropylacrylamide) (PNIPAM), subjected to an ultra-fast heating–cooling cycle. Finite element (FE) calculations were used to predict the temperature profile resulting from laser-induced heating of the polymer–aqueous system. The heating rate (∼0.6 K ps−1) deduced from FE calculations was used to heat an aqueous solution of PNIPAM consisting of 30 monomeric units (30-mer) from 285 K to 315 K. Non-equilibrium effects arising from the ultra-fast heating–cooling cycle results in a hysteresis during the coil-to-globule transition. The corresponding atomic scale conformations were characterized by monitoring the changes in the vibrational spectra, which provided a reliable metric to study the coil-to-globule transition in PNIPAM and vice-versa across the LCST. The vibrational spectra of bonds involving atoms from the oligomer backbone and the various side-groups (amide I, amide II, and the isopropyl group of PNIPAM) of the oligomers were analyzed to study the conformational changes in the oligomer corresponding to the observed hysteresis. The differences in the vibrational spectra calculated at various temperatures during heating and cooling cycles were used to understand the coil-to-globule and globule-to-coil transitions in the PNIPAM oligomer and identify the changes in the relative interactions between various atoms in the backbone and in the side groups of the oligomer with water. The shifts in the computed vibrational spectral peaks and the changes in the intensity of peaks for the different regions of PNIPAM, seen across the LCST during the heating cycle, are in good agreement with previous experimental studies. The changes in the radius of gyration (Rg) and vibrational spectra for amide I and amide II regions of PNIPAM suggest a clear coil-to-globule transition at ∼301 K during the heating cycle from 285 K to 315 K. During the heating cycle, a comparison of the vibrational spectra of isopropyl groups in PNIPAM at 285 K and 315 K suggests dehydration of the isopropyl moieties at 315 K. This implies that the oligomer–water interactions are dominant below the LCST whereas oligomer–oligomer interactions pre-dominate above the LCST. On the other hand, during the cooling cycle minor changes in the Rg and vibrational spectra of the PNIPAM oligomer in going from 315 K to 285 K indicate that the interactions between oligomer–oligomer and between the oligomer and water are less perturbed during the cooling cycle. Our simulations suggest that the observed hysteresis is a consequence of ultrafast heating–cooling kinetics, which allows insufficient relaxation times for the solvated oligomer.

 Please wait while we load your content...
Please wait while we load your content...