Electrostatic stability and encapsidation of charged nano-droplets
Abstract
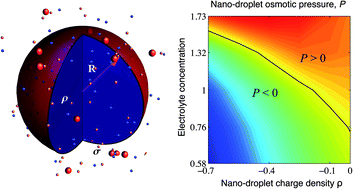
We investigate the electrostatic stability of charged droplets, modeled as permeable, charged spheres, and their encapsidation in thin, arbitrarily charged nano-shells, immersed in a neutralizing asymmetric electrolyte background. The latter consists of a small concentration of mobile multivalent counterions in a bathing solution of monovalent (positive and negative) ions. We use extensive Monte Carlo simulations to investigate the spatial distribution of multivalent counterions and the electrostatic component of their osmotic pressure on the bounding surface of the spherical nano-shells. The osmotic pressure can be negative (inward pressure), positive (outward pressure) or zero, depending on system parameters such as the charge density of the droplet, the charge density of the shell, and electrolyte screening, which thus determine the stability of the nano-container. The counter-intuitive effects of multivalent counterions comprise the increased stability of the charged droplet with larger charge density, increased stability in the case of an encapsidating shell of charge density of the same sign as the charged droplet, as well as the possibility to dispense altogether with the encapsidating shell, its confining effect being taken over by the multivalent counterions. These dramatic effects are in stark contrast to the conventional mean-field picture, which in particular implies that a more highly charged spherical droplet should be electrostatically less stable because of its larger (repulsive) self-energy.

 Please wait while we load your content...
Please wait while we load your content...