Unusual phase transition mechanism of poly(ethylene oxide) in an ionic liquid: opposite frequency shifts in C–H groups†
Abstract
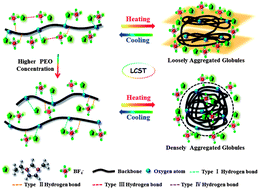
The dynamic thermally reversible phase transition behaviour of poly(ethylene oxide) (PEO) in 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]) has been investigated by calorimetric measurements (DSC) and FT-IR, in combination with perturbation correlation moving window (PCMW) and two-dimensional correlation spectroscopy (2DCOS) for the first time. A relatively high lower critical solution temperature (LCST) was observed which is different from that of conventional thermo-sensitive polymers in aqueous solution. Four types of hydrogen bonds were found in this system, among which the type I (the hydrogen bonds between the unsaturated C–H on the imidazole ring and the oxygen atoms of PEO) and type II (the hydrogen bonds between the saturated C–H on the PEO backbone and the fluorine atoms of the IL anions) ones have a more predominant influence on the phase transition. Moreover, type I plays the predominant role in both the heating and cooling process. DSC revealed that the phase transition temperature of PEO–[EMIM][BF4] solutions decreases with the increasing concentration of PEO, as well as with a decreasing scanning rate. Through PCMW, the LCST was determined to be ca. 137 °C during heating and ca. 131 °C during cooling. Finally, 2DCOS was employed to elucidate the dynamic mechanism of PEO in [EMIM][BF4], which revealed hydrogen bond disruption, chain aggregation and exclusion of IL molecules. Solutions with a low PEO concentration tend to form loosely aggregated globules, compared to the more densely aggregated globules in samples with a high PEO concentration. Additionally, it is worth mentioning that several unusual and peculiar phenomena were also observed, such as opposite frequency shifts in C–H groups, a “tail-raising” phenomenon, critical PEO concentration for the phase transition temperature, and intersection of the frequency shift curves for samples with high concentrations of PEO during cooling.

 Please wait while we load your content...
Please wait while we load your content...