Quantum chemical analysis of the thermodynamics of 2D cluster formation of 2-hydroxycarboxylic acids at the air/water interface
Abstract
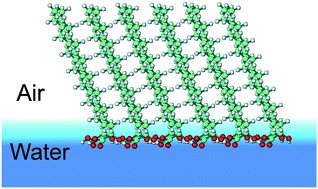
In the framework of the quantum chemical semiempirical PM3 method, the thermodynamic and structural parameters of formation and clusterization were calculated for 2-hydroxycarboxylic acids with the general formula CnH2n+1CHOHCOOH (n = 5–15). Dimers and tetramers were formed on the basis of optimized monomer structures. It was shown that the

 Please wait while we load your content...
Please wait while we load your content...