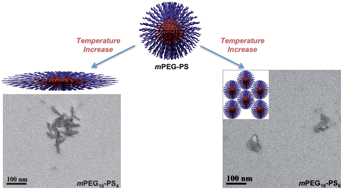
We report the thermoresponsive behavior in aqueous solution of amphiphilic diblock co-oligomers of ethylene glycol (EG) and styrene (S) obtained by reversible addition fragmentation chain transfer (RAFT) polymerization (mPEG16-b-PS2, mPEG16-b-PS4 and mPEG16-b-PS6). The block co-oligomers were obtained from the polymerization of styrene mediated by a PEG macro-RAFT agent, mPEG-PBTC. The precise control offered by RAFT polymerization over the length of the short hydrophobic chain permits tuning of the thermoresponsive behavior for all the studied methoxy poly(ethylene glycol)-block-polystyrene copolymers. mPEG16-b-PS2 was shown to self-assemble into micelles at temperatures below the lower critical solution temperature (LCST) of the block co-oligomer, whilst no such critical temperature was observed for solutions of self-assembled mPEG16-b-PS4 and mPEG16-b-PS6. mPEG16-b-PS4 self-assembles into relatively mobile spherical particles which evolve into prolate spheroids with increasing temperature, whilst mPEG16-b-PS6 forms particle clusters, which have lower mobility than the mPEG16-b-PS4 particles. This work is the first study on the aggregation behavior of such short chain lengths of mPEG-b-PS as a function of temperature, and shows how tuning the PS oligomer length permits control of the thermoresponsive behavior of mPEG-b-PS assemblies in water.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...