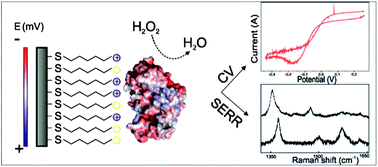
Direct electronic coupling of peroxidases with bio-compatible interfaces allows for investigation of enzyme's electro-catalytic properties that are essential in the design of bio-electronic devices. Here, a novel dye decolourising-type peroxidase from Pseudomonas putida MET94 (PpDyP) is immobilised on Ag electrodes coated with an alkanethiol self-assembled monolayer. Structural features of the active site, heterogeneous electron transfer and electro-catalytic properties of immobilised PpDyP are addressed by combination of surface enhanced spectroscopic and electrochemical approaches. They reveal that the structural integrity of the heme pocket of PpDyP is preserved upon immobilisation, the enzyme is electronically coupled to the electrode, and it exhibits efficient catalytic activity. Importantly, no significant modulation of the midpoint redox potential (Em) of the immobilised protein (Em −300 mV) is observed with respect to that in solution (Em,sol −260 mV). This study provides important structural and mechanistic insights into immobilised DyP-type peroxidase, capable of efficient decolourisation of numerous dyes, revealing PpDyP as a promising candidate for biotechnological applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...