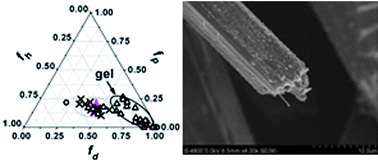
This paper proposes a simple method for predicting organogel formation, and highlights the important role played by solvent viscosity (η) and molecular size (V) in influencing the gel properties and the aggregate structures of methyl 4,6-O-(p-chlorobenzylidene)-α-D-glucopyranoside (MPBG, a model gelator) in different solvents. The research of gelation tests shows that a Teas plot derived from solubility parameters can be used to predict the behaviour of a known gelator in untested solvents. The method has successfully been tested on eight gelators reported in the literature and on untested solvents in the gelation of MPBG. The research on solvent role reveals that the aggregate morphologies of MPBG and the main factors determining the gel properties are obviously different in monohydric alcohols and aromatic hydrocarbons. According to SEM images and X-ray diffraction patterns, in monohydric alcohols with high viscosity, the self-assembly of MPBG is a diffusion-limited aggregation process resulting from solvent viscosity. Furthermore, it is found that solvent viscosity, compared with the secondary role of polar solubility parameter (δa), plays a key role in determining the morphologies of aggregates, the sol–gel phase-transition temperature (Tgel) and the gelation number (Ngel). In contrast, for aromatic hydrocarbon gels, solvent molecular size is very important in determining Tgel and Ngel although δa is the key factor.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...