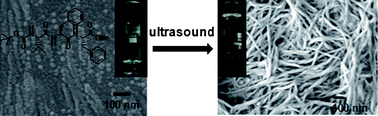
A terminally protected tripeptide Boc-Val(1)-Phe(2)-Phe(3)-OMe 1 having sequence similarity with Aβ18–20 (the central hydrophobic fragment 18–20 of the amyloid β-peptide Aβ42) forms sonication induced organogels. The peptide forms a very weak gel only in 1,2-dichlorobenzene after heating, cooling and ageing for 3 days. But ultrasound energy induces instant fibril formation and gelation in a wide range of organic solvents starting from hexane, cyclohexane, petrol, kerosene to benzene, toluene, xylene and ethanol. CD, FT-IR, NMR and wide angle X-ray scattering (WAXS) studies of the peptide 1 exhibit distinct structural changes before and after sonication. Atomic force microscopy (AFM) and field emission scanning electron microscopy (FE-SEM) of the xerogels reveal a nanofibrillar morphology, which is obtained by the sonication induced self-assembly of the gelator. These gels bind with Congo red, a physiological dye, and show a green-gold birefringence under polarized light, a characteristic feature of amyloid fibrils.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...