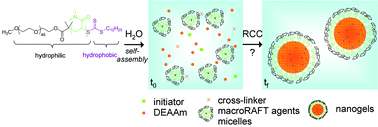
The formation of thermoresponsive poly(N,N-diethylacrylamide) (PDEAAm) nanogels via an aqueous dispersion polymerization process in the presence of poly(ethylene oxide)-b-poly(N,N-dimethylacrylamide) macromolecular reversible addition–fragmentation chain transfer agents (macroRAFT agents) was studied. The latter exhibit a hydrophobic trithiocarbonate reactive group with a dodecyl substituent, and had previously proved to act simultaneously as control agents and stabilizers in such a synthesis process (Rieger et al., J. Polym. Sci. Part A: Polym. Chem., 2009, 47, 2373). The nanogel size and stability were found to depend strongly on the chain length of the macroRAFT agents, but also on the crosslinker (N,N′-methylene bisacrylamide) and monomer concentrations. The aim of the present work was to better understand the mechanisms that govern the nanogel formation in such heterogeneous polymerization conditions performed under RAFT control, with special emphasis on the role of the macroRAFT agents. In the first part, the aqueous solution properties of the macroRAFT agents in the conditions of the dispersion polymerizations were studied by light scattering and fluorescence spectroscopy and it was found that they self-assemble to form star micelles. In the second part, the nanogel formation at different DEAAm and crosslinker concentrations was monitored by dynamic and static light scattering, and by size exclusion chromatography. It appeared that at low monomer conversion the calculated number of chains per nanogel particle was close to the aggregation number, Nagg, of the macroRAFT agent micelles. With increasing conversions, however, the number of chains clearly increased and exceeded the initial Nagg. Higher monomer concentrations hardly influenced the formation process and thus the gel particle size, whereas enhanced crosslinker concentration had a strong impact on the latter. These results strongly suggest that precursor particles are formed very rapidly at the polymerization onset and then aggregate with each other to form complex inter-crosslinked particles.

 Please wait while we load your content...
Please wait while we load your content...