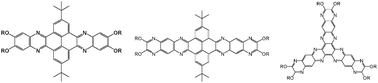
It is known that in photovoltaic applications, columnar discotic liquid crystal (LC) phases of conjugated compounds are useful to align the molecules for improving their charge mobilities. However, conjugated compounds are usually either crystalline or amorphous. For compounds to form columnar discotic LC phases, specific molecular design is required for their ordered structural packing. In our recent report, a series of conjugated compounds, 6,7,15,16-tetrakis(alkylthio)quinoxalino-[2′,3′:9,10]-phenanthro[4,5-abc]phenazine (TQPP-[SCn]4) (n = 6, 8, 10 and 12), which display p-channel characteristics, were synthesized and characterized. This series of compounds was crystalline and did not exhibit LC behavior (S. Leng, B. Wex, L. H. Chan, M. J. Graham, S. Jin, A. J. Jing, K.-U. Jeong, R. M. Van Horn, B. Sun, M. Zhu, B. R. Kaafarani and S. Z. D. Cheng, J. Phys. Chem. B, 2009, 113, 5403–5411). In order to create a columnar LC phase with the lowest free energy within a broad applicable temperature region, we specifically designed and synthesized several series of electron-deficient phenazine derivatives to disrupt the molecular crystal packing and force the compounds to enter the columnar LC phase. These phenazine derivatives were designed to control the fused rigid ring size and shape as well as the location, lengths, and chemical structures of their flexible tails. These series include a series of 2,11-bis(1-methylethyl)-6,7,15,16-tetrakis(alkoxy)quinoxalino[2′,3′:9,10]phenanthro-[4,5-abc]-phenazines (TQPP-[t-Bu]2-[OR(B)]4), a series of 2,13-bis(1-methylethyl)-7,8,18,19-tetrakis(alkoxy)pyrazino[2,3-i]pyrazino[2″,3″:6′,7′]quinoxalino[2′,3′:9,10]phenanthro[4,5-abc]-phenazines (TPPQPP-[t-Bu]2-[OR(B)]4), and a series of 3,4,11,12,19,20-hexaalkoxy-2,5,7,8,10,13,15,16,18,21,23,24-dodecaazatri-anthracenes (HDATAN-[OR]6), where R is the alkyl chain in the substituents and B represents that they are branched structures. The different phase structures and transition behaviors of these series of compounds were studied, and based on the experimental results, we can conclude that tailoring the alkyl tail size, the core size, and the core shape leads to a promising way to design molecules that exhibit the columnar LC phase. In particular, changes in alkyl tail architecture affect the phase behaviors more significantly than changes in its length.

 Please wait while we load your content...
Please wait while we load your content...