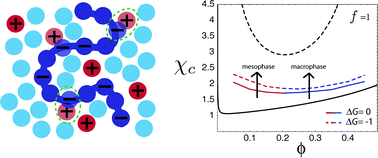
Polyelectrolytes exhibit an enhanced solubility (compared to their uncharged counterparts) due to the dissociation of counterions into the polar medium. Polyelectrolyte solutions can nevertheless become unstable, and display microphase and macrophase separation if the solvent is bad for the polyelectrolyte backbone. In the present work, we investigate theoretically the effect of counterion solubility, instead of backbone solubility, on the stability of polyelectrolyte solutions. Our results predict that the system can undergo mesophase and macrophase separation depending on the solubility of the counterion, and the effective interaction of complexed counterions with the solvent. Moreover, our studies clearly show that the phase stability of a polyelectrolyte solution can be completely determined by the type of counterion, in agreement with recent experiments. These results may be important for tailoring mesostructured materials simply by using different counterions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...