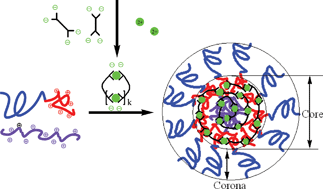
We report on capacity-controllable nanocarrier system for metal ions based on a novel kind of polymeric microemulsion. These microemulsions are formed in mixed systems of negatively charged metal-bisligand coordination polymers (cp), positively charged homopolyelectrolyte (hp), and positive-neutral diblock copolymers (dp), and are called Complex Coacervate Core microemulsions (C3-µE). The stoichiometric mixtures of cp and dp mixed system forms complex coacervate core micelles (C3M), also called polyion complex (PIC) micelles, or block ionomer complex micelles (BIC), with metal ions residing in the core as a constituent of the coordination polymers. In the presence of an additional stoichiomeric mixture of hp and cp, the micellar core swells and C3-µE are formed. In this way the amount of metal ions in a core can be increased up to 10 times, whereas by increasing the length of the dp one can only increase the amount of metal ions up to 2.5 times. The radius of the particles (Rh ≈ 140 nm) can be up to 6 times larger than the original micelles, but they still have core-shell structures. The large C3-µE particles, having densities larger than that of water, gradually sediment with time, but they do not agglomerate: they can be easily redispersed by simple shaking. C3-µEs are stable in the temperature range of 10–80 °C.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...