Oxidative chemical vapour deposition of a graphene oxide carbocatalyst on 3D nickel foam as a collaborative electrocatalyst towards the hydrogen evolution reaction in acidic electrolyte†
Abstract
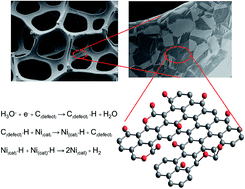
In this study, a graphene oxide (GO) carbocatalyst was synthesized as a thin film on a 3D Ni foam substrate (GO@Ni) by oxidative chemical vapour deposition (CVD) using methanol and water as precursors. The GO@Ni was used as a collaborative electrocatalyst towards the hydrogen evolution reaction (HER) in an acidic electrolyte. Notably, pure Ni metal cannot be used as an electrocatalyst in acidic media due to corrosion. The amount of water in the methanol was finely tuned to optimize the electrochemical activity of the GO@Ni for HER. The optimized GO@Ni catalyst, with a sheet resistivity of 133.48 Ω square−1, an optical band gap of 1.52 eV, and a C : O ratio of 3.89 produced using 90 : 10 V% of methanol : water, exhibits excellent and stable HER activity with an overpotential of 137 mV vs. RHE, reaching a high current density of 10 mA cm−2 and prominent electrochemical stability for up to 12 h in 0.5 M H2SO4. In addition, the CVD GO layer on the Ni foam acts as an anti-corrosion material protecting the Ni HER catalyst in the acidic environment. As a result, the GO@Ni may be a promising electrode for HER, replacing expensive Pt catalysts.


 Please wait while we load your content...
Please wait while we load your content...