Tailoring oxygen redox reactions in ionic liquid based Li/O2 batteries by means of the Li+ dopant concentration
Abstract
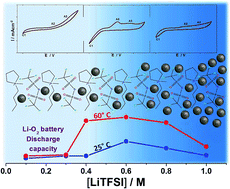
The reusability, non-volatility and non-corrosiveness of ionic liquids (ILs), as well as their ease of isolation and a large electrochemical stability window, make them an interesting choice as environmentally friendly electrolytes for metal/air batteries. ILs have been described as designer solvents as their properties and behaviour can be adjusted to suit an individual reaction need. In the framework of this study we applied a conceptually similar design approach and showed that a simple parameter such as the concentration of a Li+ dopant dramatically affects the reaction yields of Li/O2 based energy storage devices. We studied the effect of Li+ concentration from 0.1 to 1 M in a LiTFSI:PYR14TFSI ionic liquid electrolyte on the kinetics of the oxygen reduction reaction (ORR) and on the formation rate of different Li–O species at two different temperatures, finding that the discharge capacity, rates and product distribution change in a non-linear way. At 60 °C, the highest rates and up to one order of magnitude larger capacities were observed at intermediate LiTFSI concentrations, implying a complete mechanism switch from surface to volume phase mediation for Li2O2 precipitation. At room temperature the same evolution was observed, even if in this case the surface mediation remained predominant at all concentrations. These results suggest the possibility to optimise the ionic liquid based Li/O2 battery performances in terms of discharge capacity and lithium use, by tuning the temperature and alkali cation concentration.


 Please wait while we load your content...
Please wait while we load your content...