Drastic improvement in the photocatalytic activity of Ga2O3 modified with Mg–Al layered double hydroxide for the conversion of CO2 in water†
Abstract
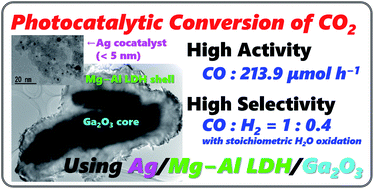
Photocatalytic conversion of CO2 to useful chemicals is a promising countermeasure against increasing concentrations of CO2 in the atmosphere as photocatalytic reactions can drive a thermodynamically uphill reaction (so-called “artificial photosynthesis”). Our group has earlier reported that Ga2O3 photocatalysts loaded with a Ag cocatalyst exhibit high activity for the conversion of CO2 to CO in water, and further modification of the catalysts with ZnGa2O4 improves the selectivity for CO over the other reduction products by suppressing H2. In this study, Ga2O3 modified with a Mg–Al layered double hydroxide (LDH) considerably enhanced not only the amount of CO evolved but also the selectivity toward CO evolution. By using 1.0 g of a Mg–Al LDH/Ga2O3 composite photocatalyst loaded with 0.25 wt% of a Ag cocatalyst, the CO formation rate and selectivity for CO were 211.7 μmol h−1 and 61.7%, respectively. Moreover, the turnover number (TON) of electrons utilized to reduce CO2 to CO was 75.4 per Ag atom during 5 h of photoirradiation.


 Please wait while we load your content...
Please wait while we load your content...