The improved ion clustering and conductivity of a di-quaternized poly(arylene ether ketone sulfone)-based alkaline fuel cell membrane†
Abstract
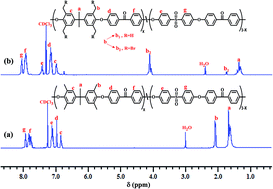
Herein, we designed a hydrophobic–hydrophilic phase-separated poly(arylene ether ketone sulfone) (PAEKS) random copolymer-based di-quaternized stable and highly conductive alkaline membrane (AM). These membranes were prepared by bromination and mono- and di-quaternization using bi-functional 1,4-diazabicyclo[2,2,2]octane (DABCO) as the quaternization agent, which showed improved ion clusters. The reported method avoids the use of hazardous choloromethyl methyl ether (CMME), and the hydrophilic–hydrophobic blocks were responsible for the formation of OH− conductive channels. Furthermore, steric hindrance (due to methyl bonded second functional group) and the micro-phase separated structure were responsible for the improved alkaline resistance and formation of ion-clusters. The structure of PAEKS was confirmed by 1H NMR and FTIR spectroscopy, whereas the molecular weight was determined by gel permeation chromatography (GPC). Transmission electron microscopy (TEM) images showed well phase-separated ionic clusters. The well optimized di-quaternized alkaline membrane with a 68% degree of bromination (di-DQP-OH (DB: 68%)) exhibited a significantly improved ion-exchange capacity (2.45 meq g−1), hydroxide ion conductivity (4.68 × 10−2 S cm−1), and activation energy (6.61 kJ mol−1) along with good thermal, mechanical, and dimensional stabilities. These properties of the AMs confirm their potential applications in alkaline fuel cells.


 Please wait while we load your content...
Please wait while we load your content...