Facile synthesis of α-aminoboronic acids from amines and potassium acyltrifluoroborates (KATs) via trifluoroborate-iminiums (TIMs)†
Abstract
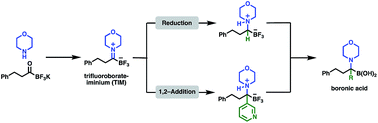
We report the facile formation of trifluoroborate-iminiums (TIMs) from potassium acyltrifluoroborates (KATs) and the transformation of TIMs to α-aminotrifluoroborates by reduction or Grignard additions. Conditions for the hydrolysis of α-aminotrifluoroborates to α-aminoboronic acids, which are important biologically active compounds, were established. This new methodology allows access to sterically demanding α-aminoboronic acids that are not easily prepared with currently available methods. This work also introduces TIMs, that can be easily prepared and handled, as a new category of functional groups that serve as precursors to valuable organic compounds.


 Please wait while we load your content...
Please wait while we load your content...