Copper mediated C–H amination with oximes: en route to primary anilines†
Abstract
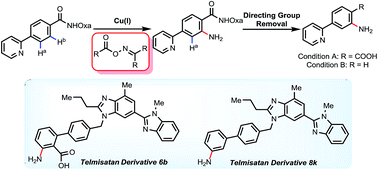
Here we report an efficient Cu(I)-mediated C–H amination reaction with oximes as amino donors to introduce NH2 groups directly. Various strongly coordinating heterocycles including quinoline, pyrimidine, pyrazine, pyrazole and triazole were tolerated well. The potential utility was further demonstrated in a late-stage modification of telmisartan (an antagonist for the angiotensin II receptor).


 Please wait while we load your content...
Please wait while we load your content...