79/81Br nuclear quadrupole resonance spectroscopic characterization of halogen bonds in supramolecular assemblies†
Abstract
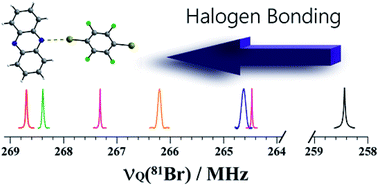
Despite the applicability of solid-state NMR to study the halogen bond, the direct NMR detection of 79/81Br covalently bonded to carbon remains impractical due to extremely large spectral widths, even at ultra-high magnetic fields. In contrast, nuclear quadrupole resonance (NQR) offers comparatively sharp resonances. Here, we demonstrate the abilities of 79/81Br NQR to characterize the electronic changes in the C–Br⋯N halogen bonding motifs found in supramolecular assemblies constructed from 1,4-dibromotetrafluorobenzene and nitrogen-containing heterocycles. An increase in the bromine quadrupolar coupling constant is observed, which correlates linearly with the halogen bond distance (dBr⋯N). Notably, 79/81Br NQR is able to distinguish between two symmetry-independent halogen bonds in the same crystal structure. This approach offers a rapid and reliable indication for the occurrence of a halogen bond, with experimental times limited only by the observation of 79/81Br NQR resonances.


 Please wait while we load your content...
Please wait while we load your content...