Dehydrogenative coupling of 4-substituted pyridines mediated by a zirconium(ii) synthon: reaction pathways and dead ends†
Abstract
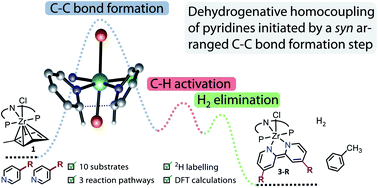
The mechanism of the reductive homocoupling of pyridine derivatives mediated by the ZrII synthon [(PNP)Zr(η6-toluene)Cl] (1) has been investigated. Selective transformation into three different types of product complexes has been observed, depending on the N-heterocyclic substrate employed: the bipyridyl complexes 3-R (R = Me, Et, tBu, Bn, Ph, CHCHPh), which are the homocoupling products, the η2-((4-dimethylamino)pyridyl) complex 4 as well as the bis(isoquinolinyl) complex 5. By deuterium labelling experiments the participation of the ligand backbone in the pyridine coupling reaction via potential cyclometallation steps was ruled out. Based on DFT modelling of the possible reaction sequences a reaction mechanism for the coupling sequence could be identified. The latter is initiated by a reductive syn C–C coupling rather than based on an initial C–H activation of the pyridine substrate.


 Please wait while we load your content...
Please wait while we load your content...