Synergistic formal ring contraction for the enantioselective synthesis of spiropyrazolones†
Abstract
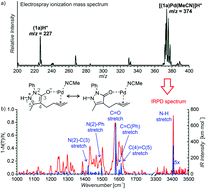
The rapid generation of molecular complexity from simple reactants is a key challenge in organic synthesis. Spiro compounds, underrepresented 3D motifs in chemical libraries, represent a challenge due to the creation of spiro quaternary carbon and the need to control the 3D shape in one step. Herein, we report the first ring contraction/formal [6 + 2] cycloaddition using synergistic Pd(0)/secondary amine catalysis, obtaining [5,5]-spiropyrazolone derivatives in excellent yields and stereoselectivities. We demonstrate that this reaction has a broad scope of early and late stage derivatization that will benefit the creation of highly valuable chemical libraries using spiropyrazolone motifs. We detected the key palladium activated intermediate in its protonated form by mass spectrometry and characterized its structure by infrared spectroscopy and DFT calculations, allowing us to propose a conceivable mechanistic pathway for this reaction.


 Please wait while we load your content...
Please wait while we load your content...