Cu-catalyzed enantioselective synthesis of tertiary benzylic copper complexes and their in situ addition to carbonyl compounds†
Abstract
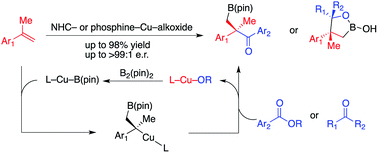
Catalytic chemo- and enantioselective generation of tertiary benzylic copper complexes from Cu–B(pin) (pin = pinacolato) additions to 1,1-disubstituted alkenes followed by in situ reactions with ketones and carboxylic acid phenol esters to construct multifunctional alkylboron compounds that contain quaternary stereogenic centers is presented. The method is distinguished by the unprecedented reaction mode of tertiary benzylic Cu complexes, allowing reaction with a wide range of carbonyl electrophiles in good yields and with high chemo-, site-, diastereo- and enantioselectivity. The catalytic protocol was performed with easily accessible chiral ligands and copper salts at ambient temperature. Functionalization of multifunctional alkylboron products provides useful building blocks that are otherwise difficult to access.


 Please wait while we load your content...
Please wait while we load your content...