Chemical proteomic profiling of protein N-homocysteinylation with a thioester probe†
Abstract
Hyperhomocysteinemia (HHcy) refers to a medical condition of abnormally high level of homocysteine (Hcy) in blood (>15 μmol L−1) and has been clinically implicated with cardiovascular diseases and neurodegenerative disorders. Excessive Hcy can be converted to a reactive thioester intermediate, Hcy thiolactone (HTL), which selectively reacts with protein lysine residues (“N-homocysteinylation”) and this non-enzymatic modification largely contributes to manifestations of HHcy. However, the proteome-wide detection of protein N-homocysteinylation remains a challenge to date. In this work, we report a chemoselective reaction to label and enrich N-homocysteinylation from complex proteome samples as inspired by native chemical ligation for protein synthesis. Alkynyl thioester probes are synthesized and the reaction is validated with small molecule and purified protein models successfully. We performed quantitative chemical proteomics to identify more than 800 N-homocysteinylated proteins as well as 304 N-homocysteinylated sites directly from HTL-treated HeLa cells. The chemical proteomics strategies will facilitate functional study of protein N-homocysteinylations in the HHcy-implicated diseases.
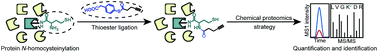


 Please wait while we load your content...
Please wait while we load your content...