Copper-catalyzed synthesis of allenylboronic acids. Access to sterically encumbered homopropargylic alcohols and amines by propargylboration†
Abstract
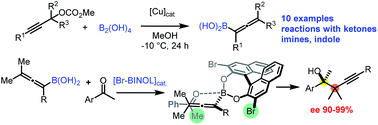
Tri- and tetrasubstituted allenylboronic acids were prepared via a new versatile copper-catalyzed methodology. The densely functionalized allenylboronic acids readily undergo propargylboration reactions with ketones and imines without any additives. Catalytic asymmetric propargylborylation of ketones is demonstrated with high stereoselectivity allowing for the synthesis of highly enantioenriched tertiary homopropargyl alcohols. The reaction is suitable for kinetic resolution of racemic allenylboronic acids affording alkynes with adjacent quaternary stereocenters.


 Please wait while we load your content...
Please wait while we load your content...