Mass and charge distributions of amyloid fibers involved in neurodegenerative diseases: mapping heterogeneity and polymorphism†
Abstract
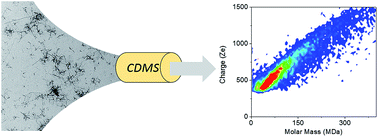
Heterogeneity and polymorphism are generic features of amyloid fibers with some important effects on the related disease development. We report here the characterization, by charge detection mass spectrometry, of amyloid fibers made of three polypeptides involved in neurodegenerative diseases: Aβ1–42 peptide, tau and α-synuclein. Beside the mass of individual fibers, this technique enables to characterize the heterogeneity and the polymorphism of the population. In the case of Aβ1–42 peptide and tau protein, several coexisting species could be distinguished and characterized. In the case of α-synuclein, we show how the polymorphism affects the mass and charge distributions.


 Please wait while we load your content...
Please wait while we load your content...