Diazomethane umpolung atop anthracene: an electrophilic methylene transfer reagent†
Abstract
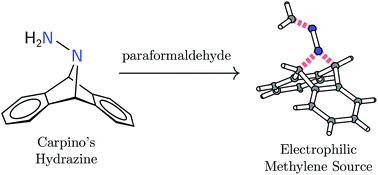
Formal addition of diazomethane's terminal nitrogen atom to the 9,10-positions of anthracene yields H2CN2A (1, A = C14H10 or anthracene). The synthesis of this hydrazone is reported from Carpino's hydrazine H2N2A through treatment with paraformaldehyde. Compound 1 has been found to be an easy-to-handle solid that does not exhibit dangerous heat or shock sensitivity. Effective umpolung of the diazomethane unit imbues 1 with electrophilicity at the methylene carbon center. Its reactivity with nucleophiles such as H2CPPh3 and N-heterocyclic carbenes is exploited for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formation with elimination of dinitrogen and anthracene. Similarly, 1 is demonstrated to deliver methylene to a nucleophilic singlet d2 transition metal center, W(ODipp)4 (2), to generate the robust methylidene complex [2
C bond formation with elimination of dinitrogen and anthracene. Similarly, 1 is demonstrated to deliver methylene to a nucleophilic singlet d2 transition metal center, W(ODipp)4 (2), to generate the robust methylidene complex [2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2]. This behavior is contrasted with that of the Wittig reagent H2CPPh3, a more traditional and Brønsted basic methylene source that upon exposure to 2 contrastingly forms the methylidyne salt [MePPh3][2
CH2]. This behavior is contrasted with that of the Wittig reagent H2CPPh3, a more traditional and Brønsted basic methylene source that upon exposure to 2 contrastingly forms the methylidyne salt [MePPh3][2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH].
CH].


 Please wait while we load your content...
Please wait while we load your content...