A unified photoredox-catalysis strategy for C(sp3)–H hydroxylation and amidation using hypervalent iodine†
Abstract
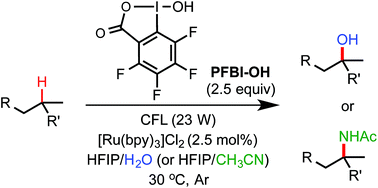
We report a unified photoredox-catalysis strategy for both hydroxylation and amidation of tertiary and benzylic C–H bonds. Use of hydroxyl perfluorobenziodoxole (PFBl–OH) oxidant is critical for efficient tertiary C–H functionalization, likely due to the enhanced electrophilicity of the benziodoxole radical. Benzylic methylene C–H bonds can be hydroxylated or amidated using unmodified hydroxyl benziodoxole oxidant Bl–OH under similar conditions. An ionic mechanism involving nucleophilic trapping of a carbocation intermediate by H2O or CH3CN cosolvent is presented.
- This article is part of the themed collections: Celebrating 100 Years of Chemistry at Nankai University, In celebration of Chinese New Year and Most downloaded articles of 2017: Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...