Efficient stabilisation of a dihydrogenphosphate tetramer and a dihydrogenpyrophosphate dimer by a cyclic pseudopeptide containing 1,4-disubstituted 1,2,3-triazole moieties†‡
Abstract
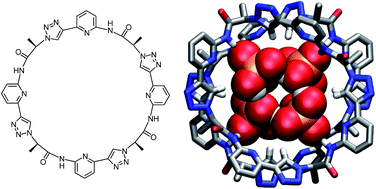
A cyclic pseudooctapeptide 2 is described containing 1,4-disubstituted 1,2,3-triazole moieties. This compound features eight converging hydrogen bond donors along the ring, namely four amide NH and four triazole CH groups, which enable 2 to engage in interactions with anions. While fully deprotonated sulfate anions exhibit only moderate affinity for 2, protonated anions such as dihydrogenpyrophosphate and dihydrogenphosphate anions are strongly bound. Complexation of the phosphate-derived anions involves sandwiching of a dihydrogenpyrophosphate dimer or a dihydrogenphosphate tetramer between two pseudopeptide rings. X-ray crystallography provided structural information, while 1H NMR spectroscopy, mass spectrometry, and isothermal titration calorimetry demonstrated that these complexes are stable in solution (2.5 vol% water/DMSO) and can even be transferred without decomposition into the gas phase. The observed high thermodynamic stabilities are attributed to the mutual reinforcement of the interactions between the individual complex components, namely, hydrogen-bonding between the anions, multiple hydrogen bonding interactions between the anion aggregates and the triazole CH and NH hydrogen bond donors of 2, and potential dispersive interactions between the closely arranged pseudopeptide rings. Pseudopeptide 2 thus represents a promising lead for the construction of phosphate receptors, whose binding selectivity makes use of the unique ability of certain anions to assemble into higher aggregates.


 Please wait while we load your content...
Please wait while we load your content...