Anomalous effect of non-alternant hydrocarbons on carbocation and carbanion electronic configurations†
Abstract
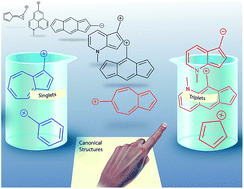
Carbocations are widely viewed to be closed-shell singlet electrophiles. Here, computations show that azulenyl-substituted carbocations have triplet ground states. This triplet ground state for azulenyl carbocations stands in striking contrast to the isomeric naphthenyl carbocation, which is a normal closed-shell singlet with a large singlet–triplet gap. Furthermore, substitution of the azulenyl carbocation can substantially alter the energy gap between the different electronic configurations and can manipulate the ground state towards either the singlet or the triplet state depending on the nature and location of the substituent. A detailed investigation into the origin of this spin state reversal, including NICS calculations, structural effects, substitution patterns, orbital analysis, and detailed linear free-energy relationships allowed us to distill a set of principles that caused these azulenyl carbocations to have such low-lying diradical states. The fundamental origin of this effect mostly centers on singlet state destabilization from increasing antiaromatic character, in combination with a smaller, but important, Baird triplet state aromatic stabilization. We find that azulene is not unique, as extension of these principles allowed us to generate simple rules to predict an entire class of analogous non-alternant carbocation and carbanion structures with low-energy or ground state diradical states, including a purely hydrocarbon triplet cation with a large singlet–triplet gap of 8 kcal mol−1. Although these ions have innocuous-looking Lewis structures, these triplet diradical ions are likely to have substantially different reactivity and properties than typical closed-shell singlet ions.


 Please wait while we load your content...
Please wait while we load your content...