3-Cyanoallyl boronates are versatile building blocks in the synthesis of polysubstituted thiophenes†
Abstract
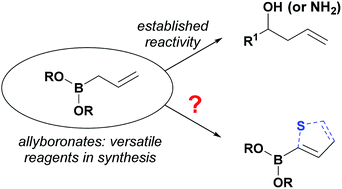
We report the preparation of hitherto unprecedented 3-cyanoallyl boronates using condensation of the parent α-boryl aldehyde and nitriles. The resulting allyl boronates have been used to generate a wide range of borylated thiophenes, which represent a valuable class of heterocycles in modern drug discovery. Subsequent Suzuki–Miyaura cross-coupling enabled the synthesis of pharmaceutically important 3,5-disubstituted aminothiophenes. Moreover, late stage functionalization gave access to borylated bromothiophene and thieno[2,3-b]pyridines.


 Please wait while we load your content...
Please wait while we load your content...