Sonogashira diversification of unprotected halotryptophans, halotryptophan containing tripeptides; and generation of a new to nature bromo-natural product and its diversification in water†
Abstract
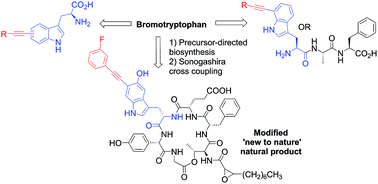
The blending together of synthetic chemistry with natural product biosynthesis represents a potentially powerful approach to synthesis; to enable this, further synthetic tools and methodologies are needed. To this end, we have explored the first Sonogashira cross-coupling to halotryptophans in water. Broad reaction scope is demonstrated and we have explored the limits of the scope of the reaction. We have demonstrated this methodology to work excellently in the modification of model tripeptides. Furthermore, through precursor directed biosynthesis, we have generated for the first time a new to nature brominated natural product bromo-cystargamide, and demonstrated the applicability of our reaction conditions to modify this novel metabolite.

 Please wait while we load your content...
Please wait while we load your content...