Cryptic post-transition state bifurcations that reduce the efficiency of lactone-forming Rh-carbenoid C–H insertions†
Abstract
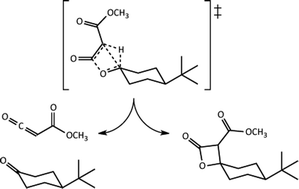
Byproducts of chemical reactions are generally thought to result from the competition between two reaction pathways, each with its own rate-determining transition state structure. We show here, however, that pathways with a single transition state structure followed by a post-transition state bifurcation may also be a source of undesired products, especially those whose appearance is unexpected. The viability of this scenario for intramolecular C–H insertion reactions affording β-lactones via Rh-carbenoid intermediates is assessed through quantum chemical calculations on potential energy surfaces and quasi-classical molecular dynamics simulations. It appears that, in these cases, the rhodium catalyst is to blame for the accessibility of a second, unintended, pathway following the transition state structure for β-lactone formation that leads to fragmentation to a ketene and carbonyl compound. If an unexpected product is formed via a post-transition state bifurcation, conventional strategies for suppressing its formation are unlikely to succeed. Guidelines for recognizing the presence of a post-transition state bifurcation are described here, along with hints at means for controlling product distributions.

 Please wait while we load your content...
Please wait while we load your content...