Ru-catalyzed sequence for the synthesis of cyclic amido-ethers†
Abstract
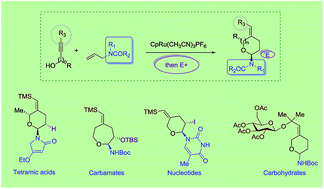
Efficient synthesis of versatile building blocks for enabling medicinal chemistry research has always challenged synthetic chemists to develop innovative methods. Of particular interest are the methods that are amenable to the synthesis of chemically distinct and diverse classes of pharmaceutically relevant motifs. Herein we report a general method for the one-pot synthesis of cyclic α-amido-ethers containing different amide functionalities including lactams, tetramic acids and amino acids. For the incorporation of the nucleotide bases, a chemo and regioselective palladium-catalyzed transformation has been developed, providing rapid access to nucleoside analogs.

 Please wait while we load your content...
Please wait while we load your content...