Multiple competing pathways for chemical reaction: drastic reaction shortcut for the self-catalytic double-helix formation of helicene oligomers†
Abstract
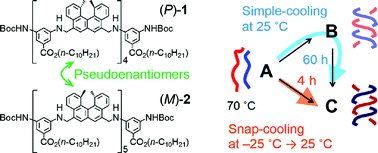
Competition among multiple pathways in a chemical reaction exhibits notable kinetic phenomena, particularly when amplification by self-catalysis is involved. A pseudoenantiomeric 1 : 1 mixture of an aminomethylene helicene (P)-tetramer and an (M)-pentamer formed enantiomeric hetero-double helices B and C in solution when random coil A was cooled. When a solution of A at 70 °C was directly cooled to 25 °C, the A-to-B reaction was predominant, then B was slowly converted to C over 60 h. The slow conversion in the A-to-B-to-C reaction was due to the formation of the hetero-double helix B, which was an off-pathway intermediate, and the slow B-to-C conversion. In contrast, when a solution of A at 70 °C was snap-cooled to −25 °C before then maintaining the solution at 25 °C, the A-to-C reaction predominated, and the formation of C was complete within 4 h. The reactions involve competition between the self-catalytic A-to-B and A-to-C pathways, where B and C catalyze the A-to-B and A-to-C reactions, respectively. Subtle differences in the initial states generated by thermal pretreatment were amplified by the self-catalytic process, which resulted in a drastic reaction shortcut.

 Please wait while we load your content...
Please wait while we load your content...