Solvatochromic fluorene-linked nucleoside and DNA as color-changing fluorescent probes for sensing interactions†
Abstract
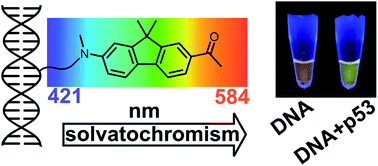
A nucleoside bearing a solvatochromic push–pull fluorene fluorophore (dCFL) was designed and synthesized by the Sonogashira coupling of alkyne-linked fluorene 8 with 5-iodo-2′-deoxycytidine. The fluorene building block 8 and labeled nucleoside dCFL exerted bright fluorescence with significant solvatochromic effect providing emission maxima ranging from 421 to 544 nm and high quantum yields even in highly polar solvents, including water. The solvatochromism of 8 was studied by DFT and ADC(2) calculations to show that, depending on the polarity of the solvent, emission either from the planar or the twisted conformation of the excited state can occur. The nucleoside was converted to its triphosphate variant dCFLTP which was found to be a good substrate for DNA polymerases suitable for the enzymatic synthesis of oligonucleotide or DNA probes by primer extension or PCR. The fluorene-linked DNA can be used as fluorescent probes for DNA–protein (p53) or DNA–lipid interactions, exerting significant color changes visible even to the naked eye. They also appear to be suitable for time-dependent fluorescence shift studies on DNA, yielding information on DNA hydration and dynamics.


 Please wait while we load your content...
Please wait while we load your content...