Neutral iodotriazoles as scaffolds for stable halogen-bonded assemblies in solution†
Abstract
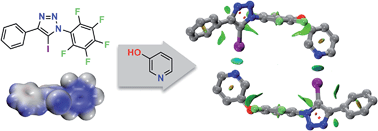
The halogen bond (XB) donor properties of neutral 1,4-diaryl-5-iodo-1,2,3-triazoles are explored using a combination of computational and experimental results and are shown to be competitive in halogen bonding efficiency with the classic pentafluoroiodobenzene XB donor. The SNAr reactivity of these donors permits the facile assembly of an iodotriazole functionalised with a 3-oxypyridine XB acceptor, thus generating a molecular scaffold capable of undergoing dimerisation through the formation of two halogen bonds. The formation of this halogen-bonded dimer is demonstrated by 1H and DOSY NMR experiments and a plausible structure generated using DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...