Electronic interactions between a stable electride and a nano-alloy control the chemoselective reduction reaction†
Abstract
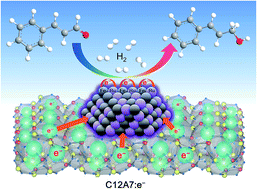
Controlling the electronic structure of heterogeneous metal catalysts is considered an efficient method to optimize catalytic activity. Here, we introduce a new electronic effect induced by the synergy of a stable electride and bimetallic nanoparticles for a chemoselective reduction reaction. The electride [Ca24Al28O64]4+·(e−)4, with extremely low work function, promotes the superior activity and selectivity of a Ru–Fe nano-alloy for the conversion of α,β-unsaturated aldehydes to unsaturated alcohols in a solvent-free system. The catalyst is easily separable from the product solution and reusable without notable deactivation. Mechanistic studies demonstrate that electron injection from the electride to the Ru–Fe bimetallic nanoparticles promotes H2 dissociation on the highly charged active metal and preferential adsorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds over C
O bonds over C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cs bond of the unsaturated aldehydes, to obtain the thermodynamically unfavorable but industrially important product.
Cs bond of the unsaturated aldehydes, to obtain the thermodynamically unfavorable but industrially important product.

 Please wait while we load your content...
Please wait while we load your content...