Stapled helical o-OPE foldamers as new circularly polarized luminescence emitters based on carbophilic interactions with Ag(i)-sensitivity†‡
Abstract
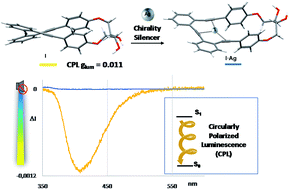
ortho-Oligo(phenylene)ethynylenes (o-OPEs) stapled with enantiopure 2,3-dihydroxybutane diethers have highly intense circular dichroism (CD) spectra and excellent circular polarized luminescence (CPL) responses (glum values up to 1.1 × 10−2), which are consistent with homochiral helically folded structures. In the presence of Ag(I), a change in the CPL emission is observed, representing the first example of CPL active small organic molecular emitters, which can be modulated by carbophilic interactions in a reversible manner.

 Please wait while we load your content...
Please wait while we load your content...